📊 Diagrams
Understanding chemical diagrams and representations
Chemical Diagrams
Chemical diagrams are visual representations that help us understand the structure, bonding, and behavior of molecules and compounds. They provide a way to visualize what we cannot see with our eyes.
Main Idea: Diagrams help us visualize molecular structures and understand chemical bonding.
Lewis Dot Structures
Lewis dot structures show the valence electrons of atoms and how they are shared or transferred in chemical bonding. They help predict molecular geometry and bonding patterns.
Rules for Drawing Lewis Structures:
1. Count Valence Electrons
- • Add up all valence electrons
- • For ions, add or subtract electrons
- • Example: H₂O = 2(1) + 6 = 8 electrons
2. Arrange Atoms
- • Central atom usually has lowest electronegativity
- • Hydrogen is never central
- • Connect atoms with single bonds
3. Distribute Electrons
- • Complete octets for all atoms
- • Hydrogen needs only 2 electrons
- • Use lone pairs if needed
4. Check Formal Charges
- • Minimize formal charges
- • Negative charge on most electronegative atom
- • Verify total charge matches ion
Example: Fluorine (F2)
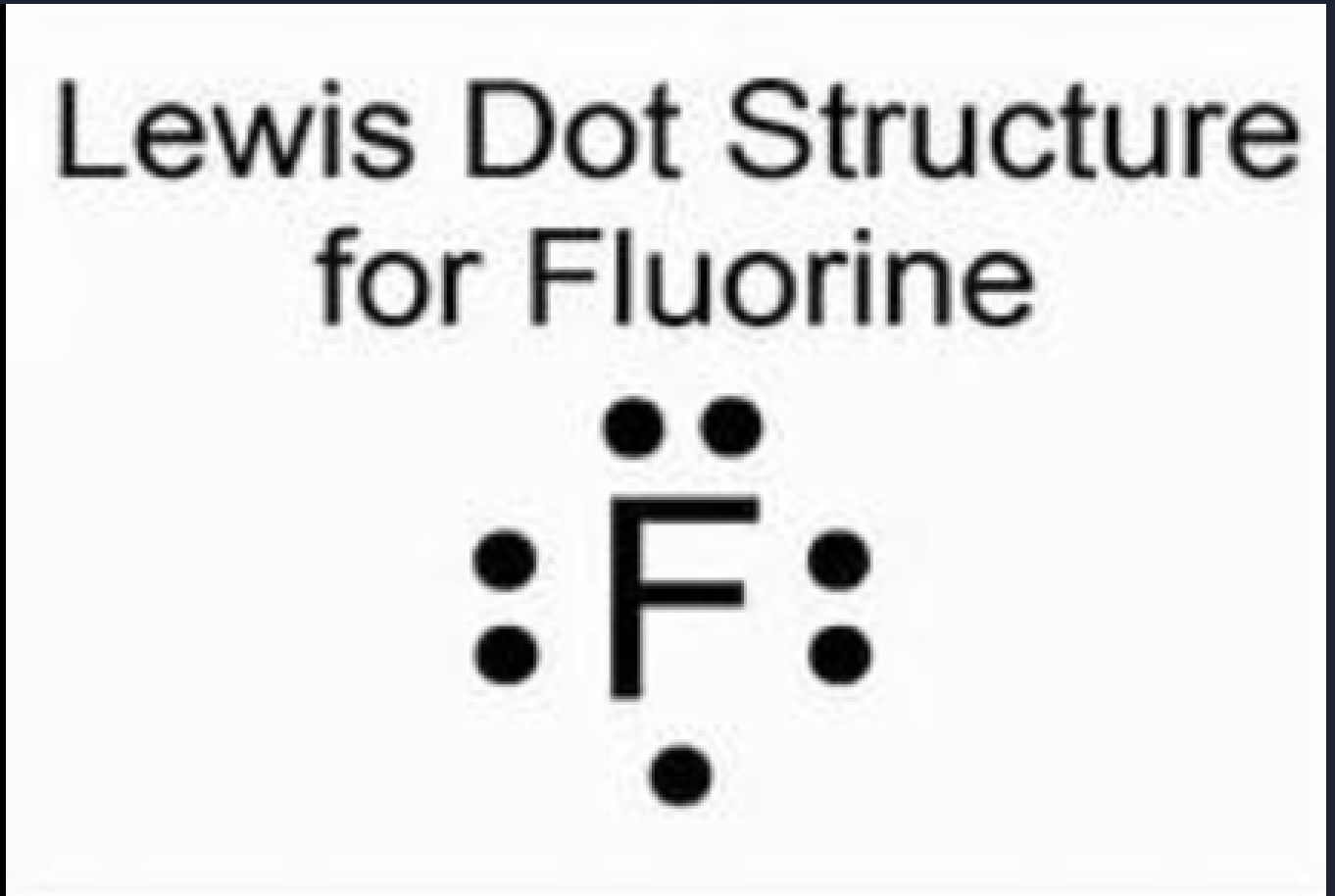
Fluorine has 7 valence electrons, its symbol is F.
Molecular Geometry
Molecular geometry describes the three-dimensional arrangement of atoms in a molecule. It's determined by the number of electron groups around the central atom.
Linear
2 electron groups, 180° bond angle
Example: CO₂, BeCl₂
Trigonal Planar
3 electron groups, 120° bond angles
Example: BF₃, SO₃
Tetrahedral
4 electron groups, 109.5° bond angles
Example: CH₄, NH₄⁺
Ball and Stick Models
Ball and stick models represent atoms as spheres and bonds as sticks. They show the three-dimensional structure and spatial arrangement of atoms in molecules.
Model Components:
Balls (Atoms)
- • Different colors for different elements
- • Size may represent atomic radius
- • Position shows spatial arrangement
Sticks (Bonds)
- • Single bonds: single sticks
- • Double bonds: double sticks
- • Triple bonds: triple sticks
Bohr-Rutherford Models
Bohr-Rutherford models show the nucleus and electron shells of atoms. They help visualize how electrons are arranged in energy levels and how atoms interact during bonding.
Model Components:
Nucleus
- • Contains protons and neutrons
- • Located at the center
- • Carries positive charge
Electron Shells
- • Concentric circles around nucleus
- • Each shell holds specific number of electrons
- • Outermost shell determines reactivity
Bohr-Rutherford Model Example
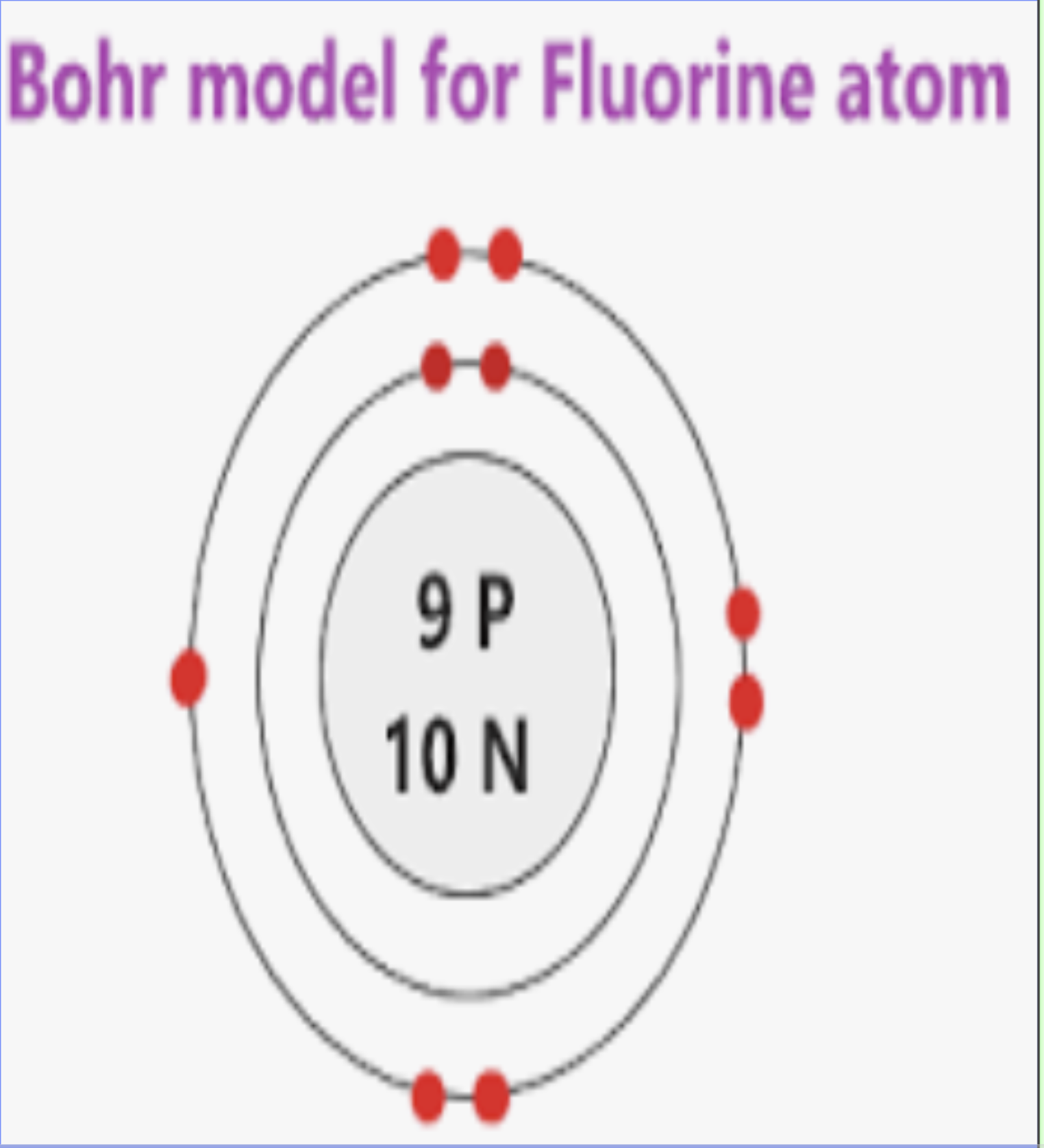
Shows the nucleus and electron shells with electrons in their energy levels
Space-Filling Models
Space-filling models show atoms as spheres that touch each other, representing the actual size and shape of molecules. They give a more realistic view of molecular volume.
Characteristics:
- • Atoms are represented as spheres with van der Waals radii
- • Spheres overlap to show covalent bonds
- • Shows the actual space occupied by the molecule
- • Useful for understanding molecular interactions
Electron Dot Diagrams
Electron dot diagrams show the valence electrons of individual atoms and how they interact to form bonds. They're simpler than Lewis structures and focus on electron sharing.
Ionic Bonding
Sodium loses an electron to chlorine, forming ions with opposite charges.
Covalent Bonding
Hydrogen atoms share electrons to form a covalent bond.
Structural Formulas
Structural formulas show the arrangement of atoms and bonds in molecules. They can be condensed, expanded, or skeletal depending on the level of detail needed.
Condensed Formula
CH₃CH₂OH (ethanol)
Expanded Formula
H—C—C—O—H (shows all bonds)
Skeletal Formula
Lines represent carbon-carbon bonds, vertices are carbon atoms
Lazy Read
- • Lewis structures show valence electrons and bonding patterns
- • Molecular geometry depends on electron groups around central atom
- • Ball and stick models show 3D structure with bonds as sticks
- • Bohr-Rutherford models show nucleus and electron shells
- • Space-filling models show actual molecular volume
- • Electron dot diagrams focus on electron sharing
- • Structural formulas show atom arrangement in different detail levels